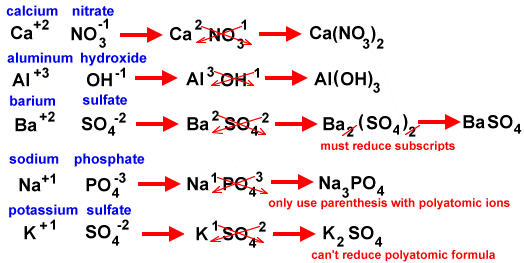
To get the charges to balance out, we add subscripts to show the number of each type of ion in the compound. If the charges are already equal, but opposite, they will be in a 1:1 ratio. So, Na+1 and Cl -1 combine to form NaCl. Al+3 and N-3 combine to form AlN. However, if the charges are NOT equal (they will still be opposite because like charges won't attract), we use the criss-cross method. For the criss-cross method, we take the number for one element's charge and cross it over to become the other element's subscript.
 |
| Image from here |
Creating ionic bonds using polyatomic ions would be similar. However, we would have the charge of the entire polyatomic ion cancel out with the other charge. If more the subscript for the polyatomic ion would be more than 1, we would put parenthesis around the polyatomic ion and then write the new subscript. You cannot change anything about the polyatomic ion. It must stay together as if it were a single ion because that's how polyatomic ions work.
 |
| Image from here |
No comments:
Post a Comment