First, a simple bond between a metal that is NOT a transition metal and a nonmetal. The charges of these elements is always the same, so we don't have to do anything fancy with the names. We simply write the name of the positive ion first. Then, we write the negative ion and change the ending to -ide. Simple! For example, NaCl would be sodium chloride; MgBr2 would be magnesium bromide. There is no need to mention the subscript 2 because it only tells us that we need 2 Br- ions to balance out the Mg+2 ion.
Second, bonds involving transition metals and nonmetals can be a little trickier. This is because many of the transition metals can have different charges. Iron can have a +2 or +3 charge! That means iron can bond with oxygen in two ways. It can be FeO or Fe2O3! So, how are you supposed to clarify that it the name? Simple, we write the charge of iron in parenthesis. FeO would be iron (II) oxide (notice the negative ion still ends in -ide) and Fe2O3 would be iron (III) oxide.
How do you figure out which iron is which using the formula? How about a little reverse formula writing :) Now, FeO has iron and oxygen bonded in a 1:1 ratio; in class we said that is the ratio is 1:1 that means the charges are equal and opposite. We know that oxygen always forms a -2 ion (because it is in family 6 and gains two electrons); that means that iron has to be a +2 ion (opposite and equal). On the other hand, Fe2O3 is a 2:3 ratio, so we'll do the criss-cross method in reverse.
Fe2O3
Fe+3 O-2
Since this iron had to have a +3 charge, we write it as iron (III) oxide.
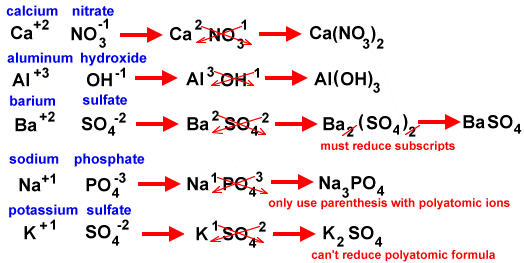
Third, the bond can involve polyatomic ions, which are atoms bonded together and acting as a single ion. Any compound involving polyatomic ions is named with the positive ion first and the negative ion second (as all are and as the formulas are written). BUT, the name of the polyatomic ion is NEVER changed. If the formula is MgSO4 then the name is magnesium sulfate. Mg+2 is the positive ion of magnesium and SO4 -2 is sulfate. There is no change to the ending of the negative ion because it is a polyatomic ion. We haven't had any of these for you to name in class and you aren't expected to memorize the names of polyatomic ions.